
Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>



































| Cat. No. | Species | Product Description | Structure | Purity | Feature |
|---|---|---|---|---|---|
| FUN-MY2098 | Mouse | Monoclonal Anti-Hendra virus F glycoprotein Antibody, Human IgG1 (3F4) (MALS verified) |
|
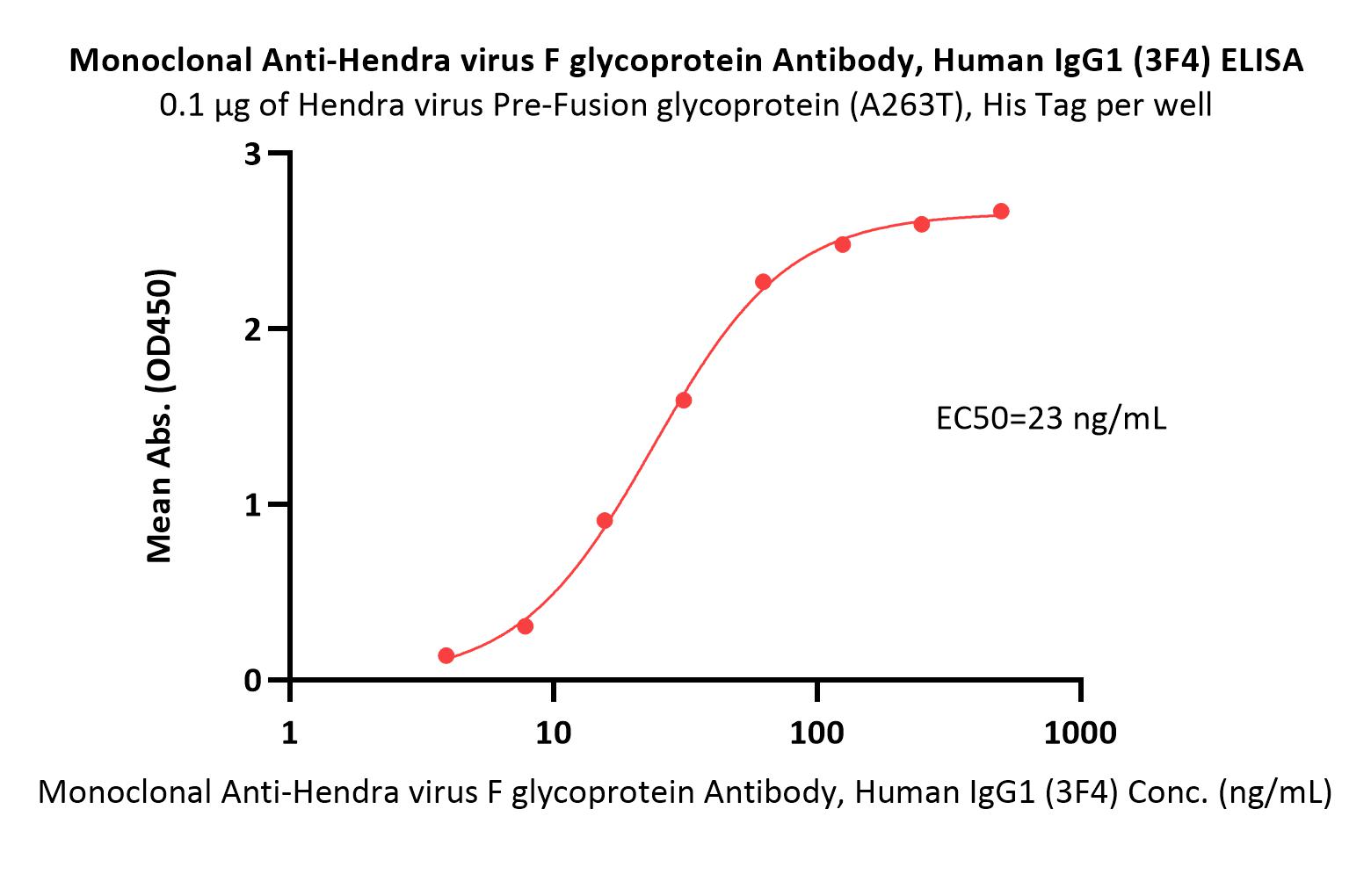
|
|
| FUN-MY2097 | Mouse | Monoclonal Anti-Hendra virus F glycoprotein Antibody, Human IgG1 (1A7) (MALS verified) |
|
|

Immobilized Hendra virus Pre-Fusion glycoprotein (A263T), His Tag (Cat. No. FUN-H52H4) at 1 μg/mL (100 μL/well) can bind Monoclonal Anti-Hendra virus F glycoprotein Antibody, Human IgG1 (1A7) (Cat. No. FUN-MY2097) with a linear range of 0.6-9.8 ng/mL (QC tested).
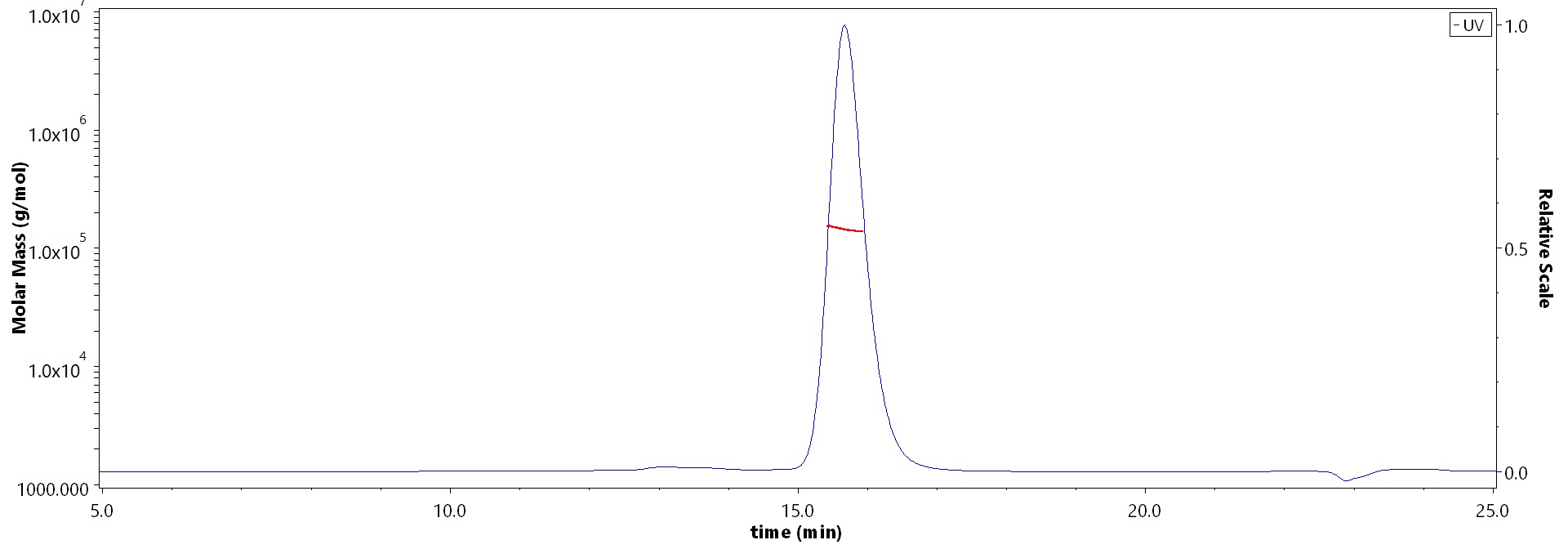
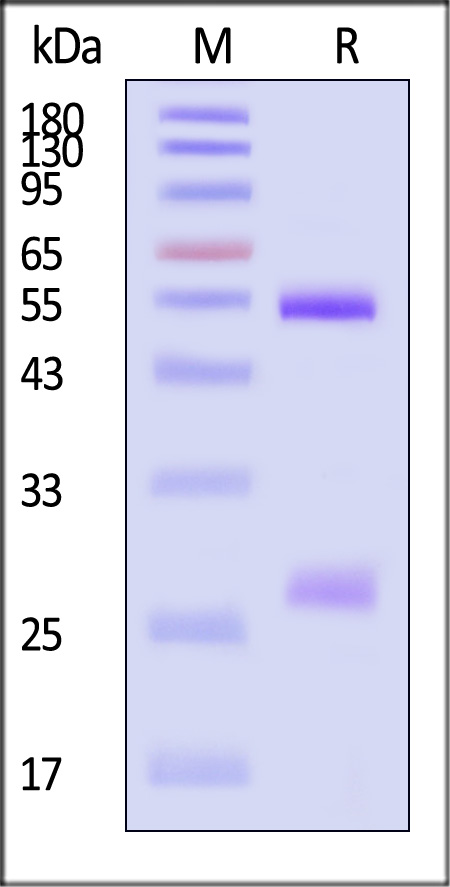
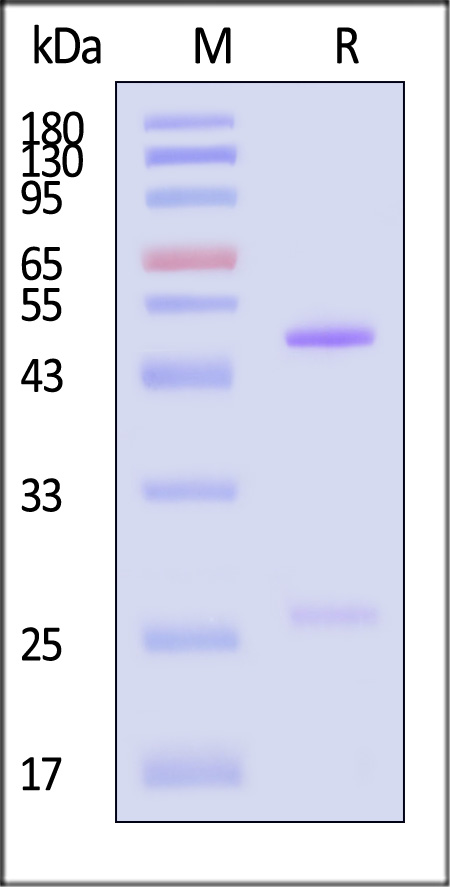
The purity of Monoclonal Anti-Hendra virus F glycoprotein Antibody, Human IgG1 (3F4) (Cat. No. FUN-MY2098) is more than 90% and the molecular weight of this protein is around 135-160 kDa verified by SEC-MALS.
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Nirsevimab | Anti-RSV-mAb-D25; SP-0232; MEDI-8897; Anti-RSV MAb-YTE | Approved | Aimm Therapeutics, Sanofi, MedImmune Inc | Beyfortus, 乐唯初 | EU | Respiratory Syncytial Virus Infections | Sanofi Winthrop Industrie SA | 2022-10-31 | Lower Respiratory Tract Infections; Respiratory Syncytial Virus Infections | Details |
| Palivizumab | ABT-315; MEDI-493 | Approved | Abbvie Inc, Medimmune Llc | Synagis | United States | Respiratory Syncytial Virus Infections | Swedish Orphan Biovitrum Ab (Publ) | 1998-06-19 | Bronchiolitis; Bronchopulmonary Dysplasia; Respiratory Syncytial Virus Infections; Heart Defects, Congenital; Infant, Premature, Diseases; Premature Birth | Details |
| Respiratory syncytial virus immune globulin | RSV-IGIV | Approved | Medimmune | RespiGam | United States | Respiratory Syncytial Virus Infections | null | 1996-01-01 | Respiratory Syncytial Virus Infections | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Human Respiratory Syncytial Virus Monoclonal Antibody | TNM-001 | Phase 3 Clinical | Zhuhai Trinomab Pharmaceutical Co Ltd | Lower Respiratory Tract Infections; Respiratory Syncytial Virus Infections | Details |
| CPI-RSV-F Vaccine | BLB-201 | Phase 2 Clinical | Blue Lake Biotechnology Inc | Respiratory Syncytial Virus Infections | Details |
| Ziresovir | RO-0529; AK-0529 | Phase 1 Clinical | F. Hoffmann-La Roche Ltd | Respiratory Syncytial Virus Infections | Details |
| GR-2102 | GR2102; GR-2102 | Phase 1 Clinical | Genrix (Shanghai) Biopharmaceutical Co Ltd, Chongqing Zhixiang Jintai Biopharmaceutical Co Ltd | Respiratory Syncytial Virus Infections | Details |
| Palivizumab biosimilar (mAbxience) | Phase 1 Clinical | Mabxience Sa | Details | ||
| RSM-01 | Phase 1 Clinical | Bill & Melinda Gates Medical Research Institute | Respiratory Syncytial Virus Infections | Details |
This web search service is supported by Google Inc.







